
Each country has its own standards for the control of HMOs (human milk oligosaccharides), which means that HMOs must meet the requirements of the country's standards in order to be marketed in that country. In China, HMOs is managed according to new varieties of nutritional fortifiers, while the United States manages HMOs as a Generally Recognized as Safe (GRAS) substance, while the European Food Safety Agency (hereinafter referred to as the "EU") and the Australian and New Zealand Food Standards Agency (hereinafter referred to as "ANZ") manage HMOs as a new food ingredient.
Types approved by HMOs
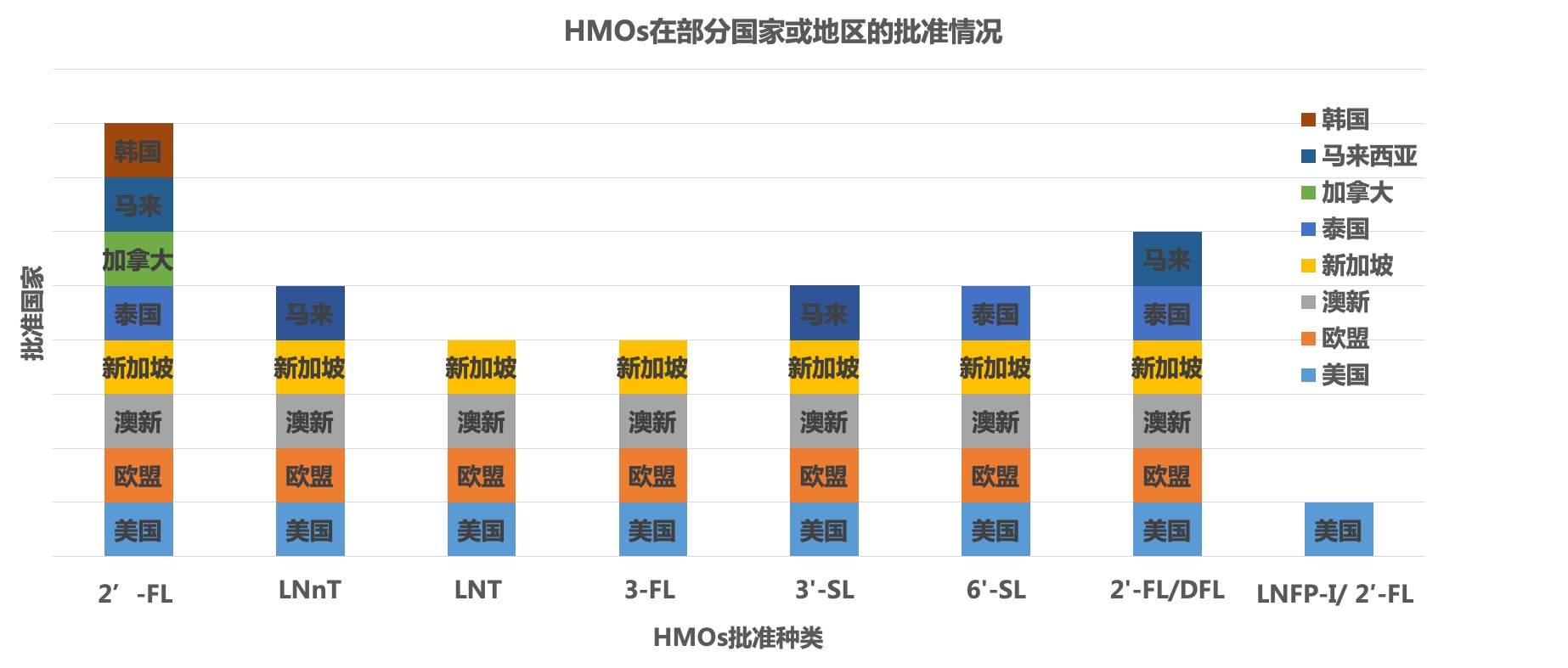
Application scope approved by HMOs
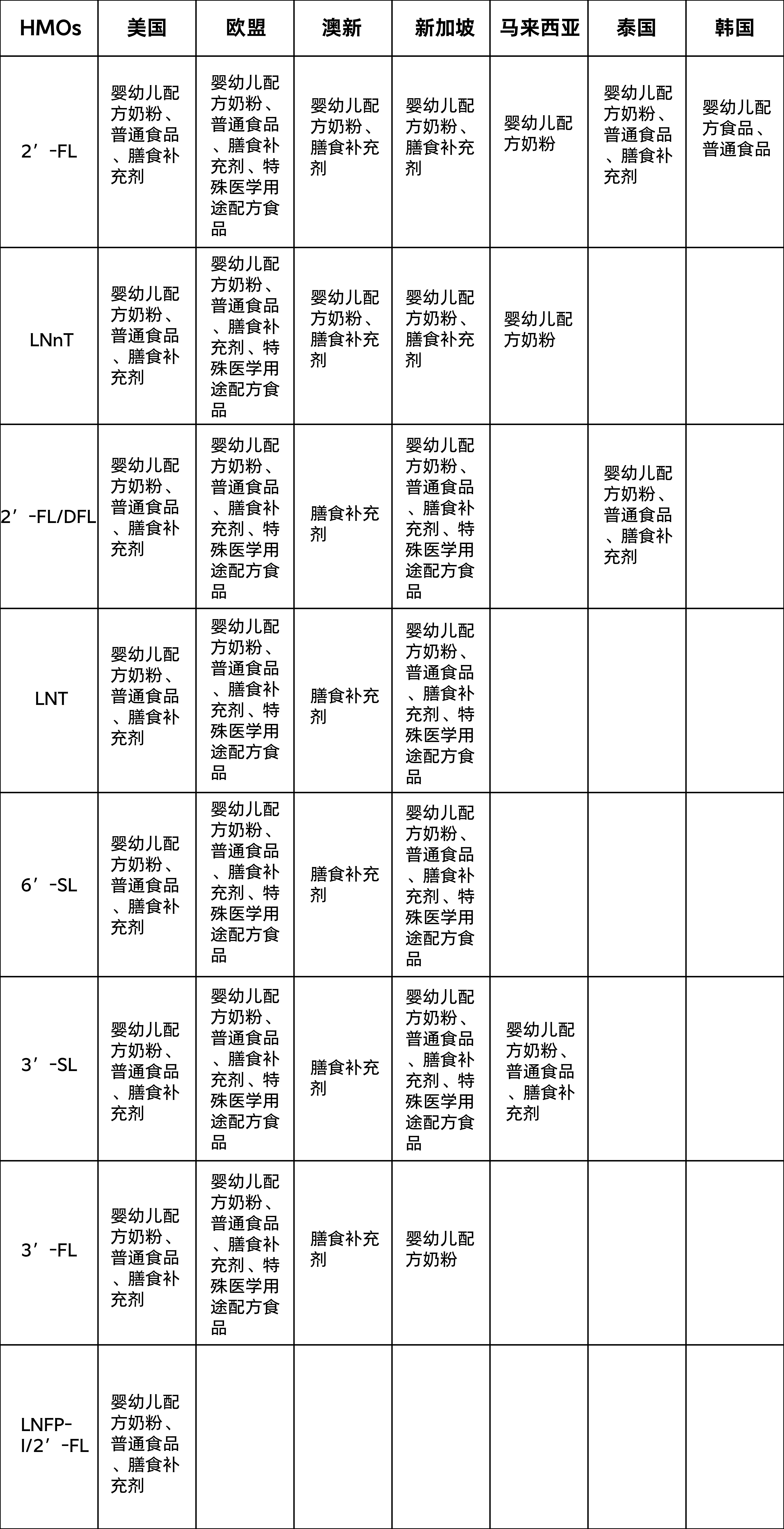
Approved usage of HMOs
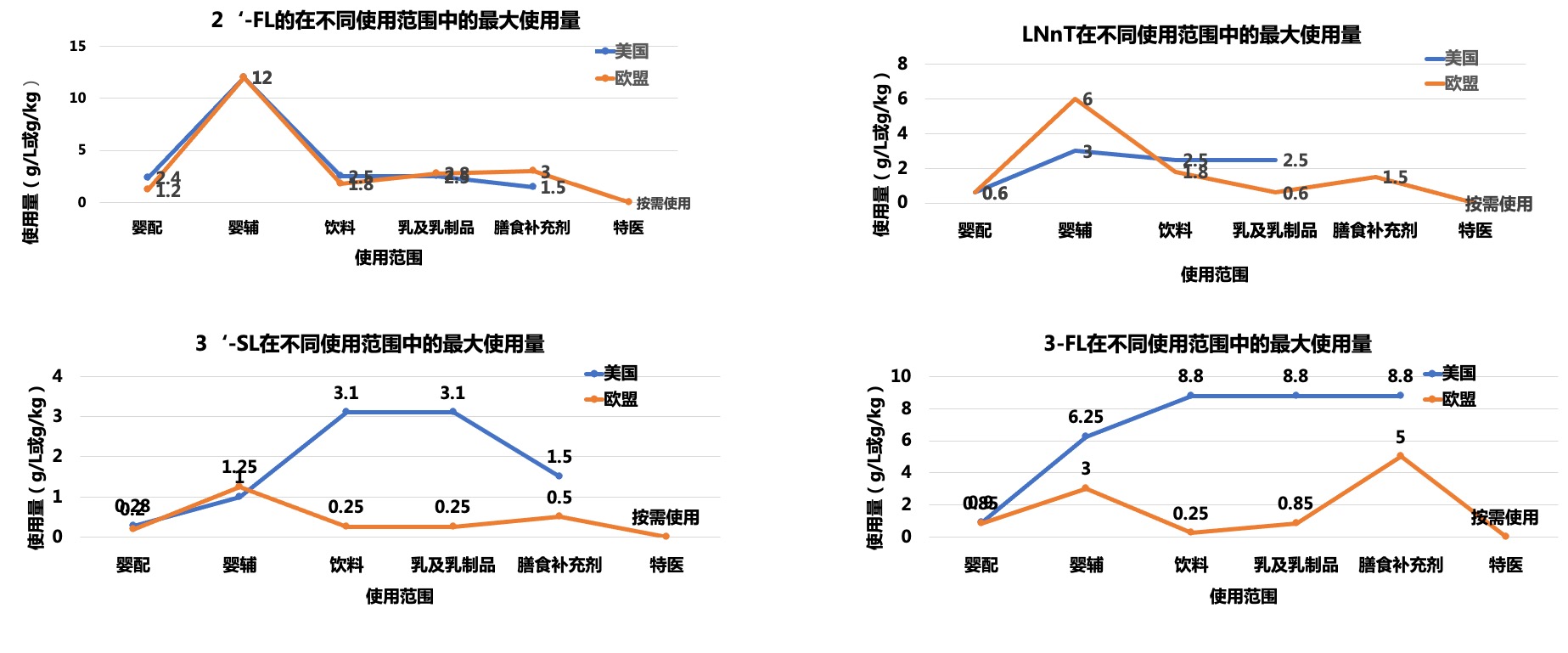
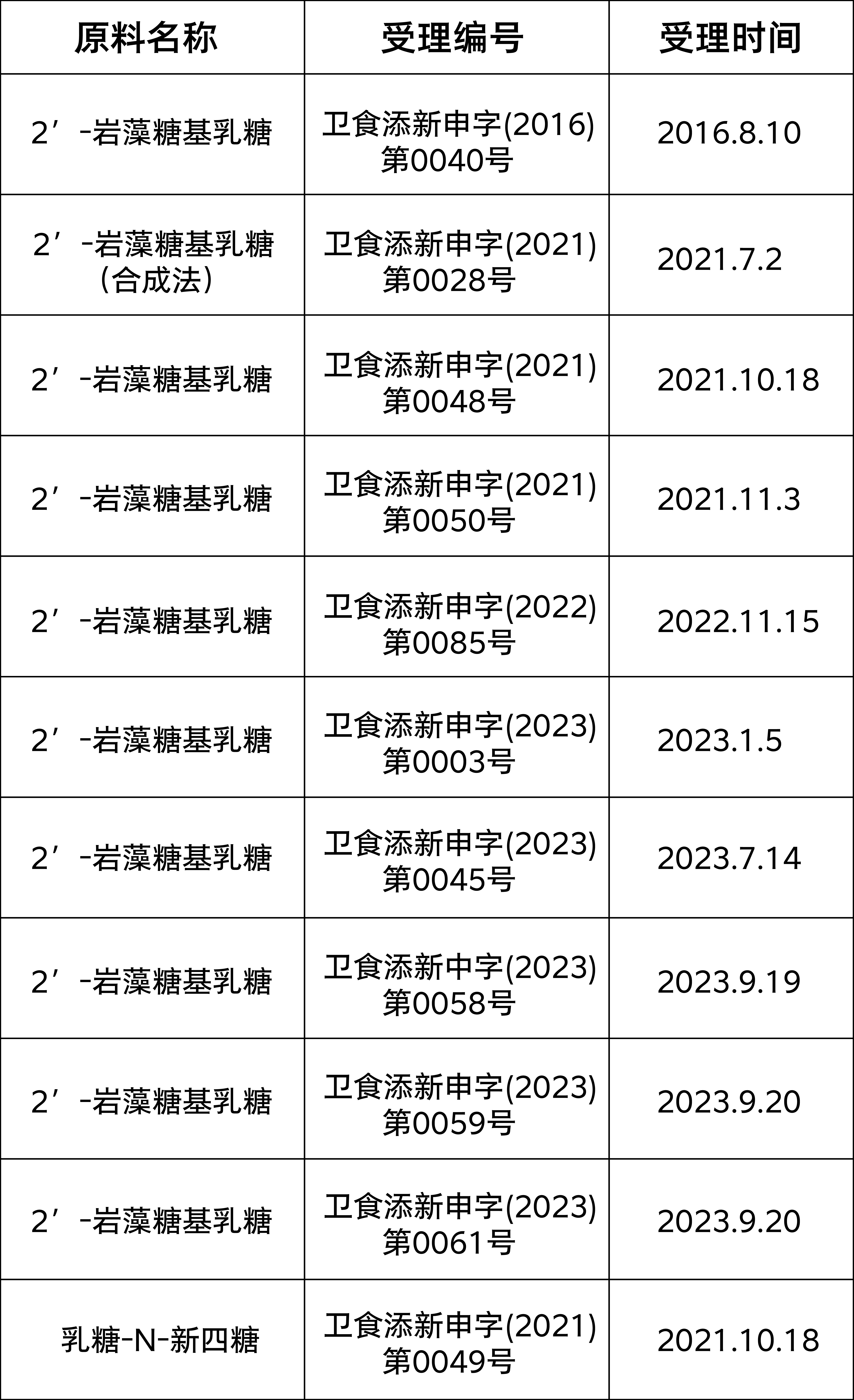
In China, in order to ensure the safety of HMOs used in infant formula food, enterprises and professional institutions including Hongmu Biotechnology have conducted a lot of work and evaluation verification on the safety of HMOs used in infant formula food, including the safety evaluation of genetically engineered bacteria involved in HMO production and the safety evaluation of HMOs themselves. The safety evaluation verification has laid the foundation for successful approval in China.
As of now, 2 '- fucosyllactose has been released for acceptance information 10 times, and lactose-N-neotetraose has been released once. 2' - fucosyllactose, as the HMOs variety with the highest content, is also the most popular.
On October 7, 2023, the National Health Commission issued a notice on 15 "three new foods" including peach gum, which includes two HMOs substances 2 '- fucosyllactose and lactose-N-neotetraose. Its application scope can be used as a food nutritional enhancer in formulated milk powder (limited to infant milk powder), infant formula food, and special medical infant formula food. The approved doses of 2 '- FL and LNnT for immediate consumption are 0.7~2.4 g/L and 0.2~0.6 g/L, respectively.
The official approval of HMOs will inject new momentum into the innovation and upgrading of products in China's food industry, which is of great significance for supporting early life nutrition research, promoting high-quality development and industrial upgrading in areas such as infant formula food and special medical purpose formula food in China.
In October 2023, Synaura 2 '- FL (2′-fucosyllactose) won the first batch of the Health Commission and became the only local approved enterprise in China. It has a new milestone significance in the research and application of HMOs. Synaura is willing to carry the local innovation mission, continue to promote the vigorous development of local biological synthesis technology, and help China become the flag benchmark of the fourth industrial wave.
In October 2023, Synaura 2 '- FL (2′-fucosyllactose) won the first batch of the Health Commission and became the only local approved enterprise in China. It has a new milestone significance in the research and application of HMOs. Synaura is willing to carry the local innovation mission, continue to promote the vigorous development of local biological synthesis technology, and help China become the flag benchmark of the fourth industrial wave.
